Engineers are constantly looking for ways to create materials that are both stronger and lighter, especially for use in extreme conditions like those found in jet engines and spacecraft. Fadi Abdeljawad, an associate professor of materials science and engineering at Lehigh University, believes that the key to unlocking these innovative materials lies in the tiny boundaries where atoms in crystals meet.
Abdeljawad, along with researchers from the U.S. Department of Energy’s Center for Integrated Nanotechnologies (CINT), is delving into how these minuscule boundaries impact the properties of nanomaterials. Nanocrystals, which are structures about 1/10,000th the width of a human hair, stack together to form most engineering materials. It is at the points where these crystals meet that the behavior of the material is determined.
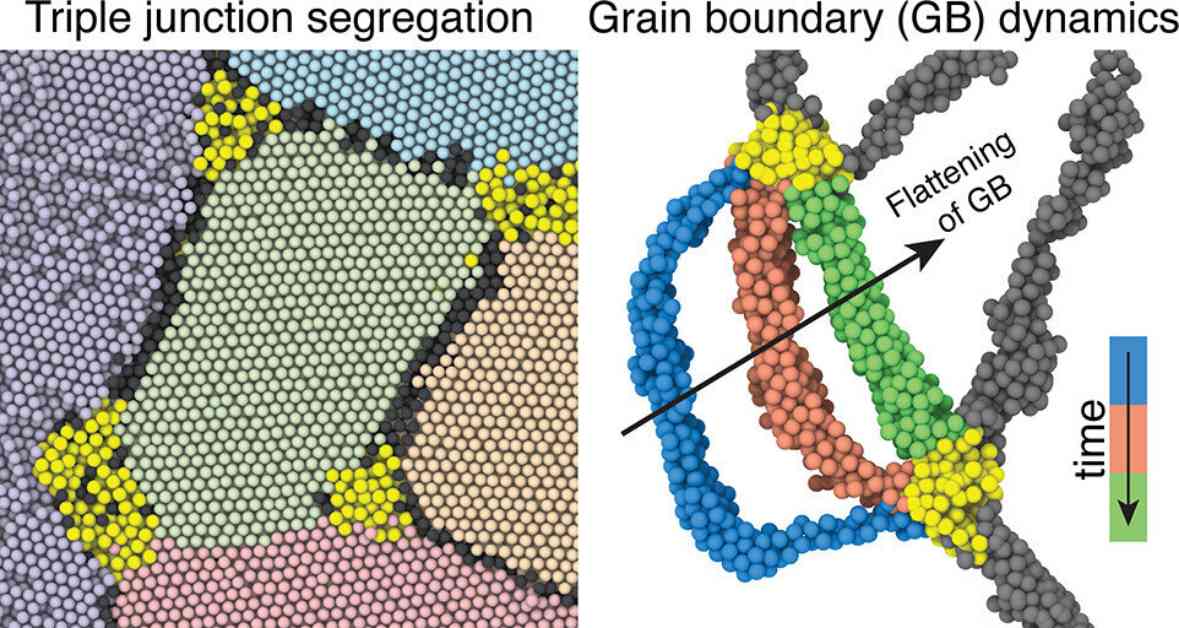
A recent study published in Nano Letters, titled “Triple Junction Segregation Dominates the Stability of Nanocrystalline Alloys,” sheds light on the significance of triple junctions in nanomaterials. These junctions, where three nanocrystals converge, play a crucial role in maintaining the stability of materials at high temperatures.
The researchers found that by adding specific atoms to create an alloy, these atoms tend to gather at triple junctions. This chemical segregation helps prevent the growth of grains within the material, thereby preserving its strength over time. For example, placing gold atoms strategically at triple junctions in a platinum nanomaterial allowed the material to remain stable under high-temperature conditions.
Understanding this process enables scientists to design more resilient nanocrystalline alloys by selecting elements that will stabilize the material at its junctions. This knowledge is particularly valuable for industries like aerospace and energy, where durability at elevated temperatures is essential.
Abdeljawad’s computational studies, in collaboration with CINT’s advanced tools and expertise, have paved the way for these discoveries. By simulating the complex atomic arrangements within materials, researchers can tailor features at the nanoscale to engineer novel materials effectively.
This collaborative effort highlights the power of teamwork in advancing scientific knowledge and technological innovations. By combining computational models with experimental validation, researchers can push the boundaries of materials science and create new possibilities for extreme condition applications.
In conclusion, the exploration of atom meeting points and triple junctions in nanomaterials offers a promising avenue for developing materials that can withstand extreme conditions while maintaining their strength and stability. This interdisciplinary approach to materials science opens up exciting opportunities for future research and innovation in engineering applications.